
SL Paper 2
Define the term isotopes.
A sample of silicon contains three isotopes.
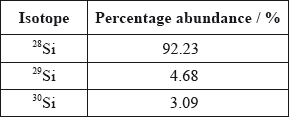
Calculate the relative atomic mass of silicon using this data.
Describe the structure and bonding in silicon dioxide and carbon dioxide.
Draw the Lewis structure of NH3, state its shape and deduce and explain the H–N–H bond angle in \({\text{N}}{{\text{H}}_{\text{3}}}\).
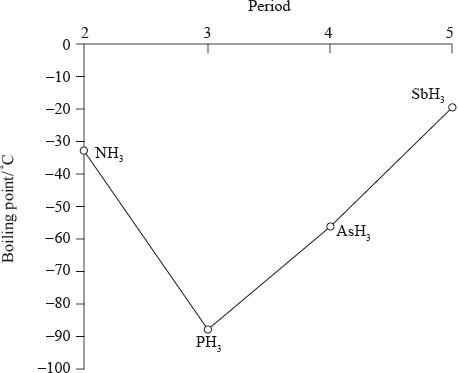
The graph below shows the boiling points of the hydrides of group 5. Discuss the variation in the boiling points.
Explain, using diagrams, why CO and \({\text{N}}{{\text{O}}_{\text{2}}}\) are polar molecules but \({\text{C}}{{\text{O}}_{\text{2}}}\) is a non-polar molecule.
If white anhydrous copper(II) sulfate powder is left in the atmosphere it slowly absorbs water vapour giving the blue pentahydrated solid.
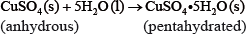
It is difficult to measure the enthalpy change for this reaction directly. However, it is possible to measure the heat changes directly when both anhydrous and pentahydrated copper(II) sulfate are separately dissolved in water, and then use an energy cycle to determine the required enthalpy change value, \(\Delta {H_{\text{x}}}\), indirectly.
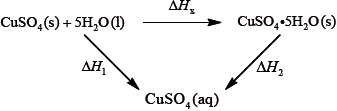
To determine \(\Delta {H_1}\) a student placed 50.0 g of water in a cup made of expanded polystyrene and used a data logger to measure the temperature. After two minutes she dissolved 3.99 g of anhydrous copper(II) sulfate in the water and continued to record the temperature while continuously stirring. She obtained the following results.
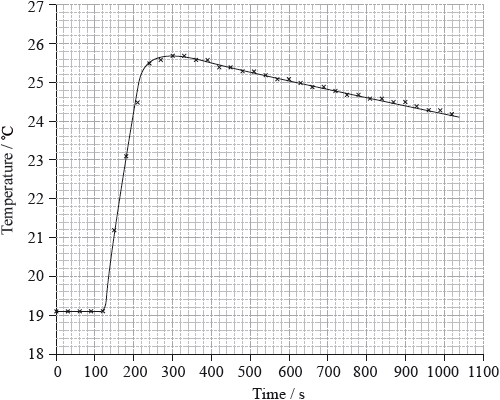
To determine \(\Delta {H_2}\), 6.24 g of pentahydrated copper(II) sulfate was dissolved in 47.75 g of water. It was observed that the temperature of the solution decreased by 1.10 °C.
The magnitude (the value without the \( + \) or \( - \) sign) found in a data book for \(\Delta {H_{\text{x}}}\) is \({\text{78.0 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Calculate the amount, in mol, of anhydrous copper(II) sulfate dissolved in the 50.0 g of water.
Determine what the temperature rise would have been, in °C, if no heat had been lost to the surroundings.
Calculate the heat change, in kJ, when 3.99 g of anhydrous copper(II) sulfate is dissolved in the water.
Determine the value of \(\Delta {H_1}{\text{ in kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Calculate the amount, in mol, of water in 6.24 g of pentahydrated copper(II) sulfate.
Determine the value of \(\Delta {H_2}\) in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Using the values obtained for \(\Delta {H_1}\) in (a) (iv) and \(\Delta {H_2}\) in (b) (ii), determine the value for \(\Delta {H_{\text{x}}}\) in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Calculate the percentage error obtained in this experiment. (If you did not obtain an answer for the experimental value of \(\Delta {H_{\text{x}}}\) then use the value \({\text{70.0 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), but this is not the true value.)
The student recorded in her qualitative data that the anhydrous copper(II) sulfate she used was pale blue rather than completely white. Suggest a reason why it might have had this pale blue colour and deduce how this would have affected the value she obtained for \(\Delta {H_{\text{x}}}\).
Iron tablets are often prescribed to patients. The iron in the tablets is commonly present as iron(II) sulfate, \({\text{FeS}}{{\text{O}}_{\text{4}}}\).
Two students carried out an experiment to determine the percentage by mass of iron in a brand of tablets marketed in Cyprus.
Experimental Procedure:
• The students took five iron tablets and found that the total mass was 1.65 g.
• The five tablets were ground and dissolved in \({\text{100 c}}{{\text{m}}^{\text{3}}}\) dilute sulfuric acid, \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\). The solution and washings were transferred to a \({\text{250 c}}{{\text{m}}^{\text{3}}}\) volumetric flask and made up to the mark with deionized (distilled) water.
• \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of this \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) solution was transferred using a pipette into a conical flask. Some dilute sulfuric acid was added.
• A titration was then carried out using a \(5.00 \times {10^{ - 3}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) standard solution of potassium permanganate, \({\text{KMn}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\). The end-point of the titration was indicated by a slight pink colour.
The following results were recorded.
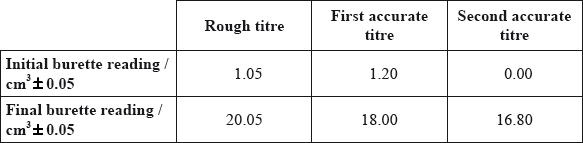
This experiment involves the following redox reaction.
\[{\text{5F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{MnO}}_4^ - {\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{5F}}{{\text{e}}^{3 + }}{\text{(aq)}} + {\text{M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\]
When the \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) solution was made up in the \({\text{250 c}}{{\text{m}}^{\text{3}}}\) volumetric flask, deionized (distilled) water was added until the bottom of its meniscus corresponded to the graduation mark on the flask. It was noticed that one of the two students measured the volume of the solution from the top of the meniscus instead of from the bottom. State the name of this type of error.
State what is meant by the term precision.
When the students recorded the burette readings, following the titration with KMnO4 (aq),the top of the meniscus was used and not the bottom. Suggest why the students read the top of the meniscus and not the bottom.
Define the term reduction in terms of electrons.
Deduce the oxidation number of manganese in the \({\text{MnO}}_{\text{4}}^ - {\text{(aq)}}\) ion.
Determine the amount, in mol, of \({\text{MnO}}_{\text{4}}^ - {\text{(aq)}}\), used in each accurate titre.
Calculate the amount, in mol, of \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) ions in \({\text{250 c}}{{\text{m}}^{\text{3}}}\) of the solution.
Determine the total mass of iron, in g, in the \({\text{250 c}}{{\text{m}}^{\text{3}}}\) solution.
Determine the percentage by mass of iron in the tablets.
One titration was abandoned because a brown precipitate, manganese(IV) oxide, formed. State the chemical formula of this compound.
Ethanol is used as a component in fuel for some vehicles. One fuel mixture contains 10% by mass of ethanol in unleaded petrol (gasoline). This mixture is often referred to as Gasohol E10.
Assume that the other 90% by mass of Gasohol E10 is octane. 1.00 kg of this fuel mixture was burned.
\[\begin{array}{*{20}{l}} {{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH(l)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - 1367{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{{\text{C}}_8}{{\text{H}}_{18}}{\text{(l)}} + {\text{12}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{8C}}{{\text{O}}_2}{\text{(g)}} + {\text{9}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - 5470{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Calculate the mass, in g, of ethanol and octane in 1.00 kg of the fuel mixture.
Calculate the amount, in mol, of ethanol and octane in 1.00 kg of the fuel mixture.
Calculate the total amount of energy, in kJ, released when 1.00 kg of the fuel mixture is completely burned.
If the fuel blend was vaporized before combustion, predict whether the amount of energy released would be greater, less or the same. Explain your answer.
Aspirin, one of the most widely used drugs in the world, can be prepared according to the equation given below.
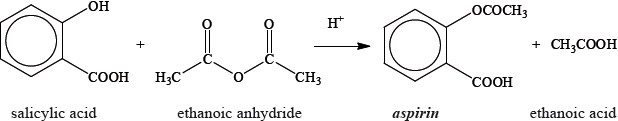
A student reacted some salicylic acid with excess ethanoic anhydride. Impure solid aspirin was obtained by filtering the reaction mixture. Pure aspirin was obtained by recrystallization. The following table shows the data recorded by the student.

State the names of the three organic functional groups in aspirin.
Determine the amount, in mol, of salicylic acid, \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{\text{(OH)COOH}}\), used.
Calculate the theoretical yield, in g, of aspirin, \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{\text{(OCOC}}{{\text{H}}_{\text{3}}}{\text{)COOH}}\).
Determine the percentage yield of pure aspirin.
State the number of significant figures associated with the mass of pure aspirin obtained, and calculate the percentage uncertainty associated with this mass.
Another student repeated the experiment and obtained an experimental yield of 150%. The teacher checked the calculations and found no errors. Comment on the result.
The following is a three-dimensional computer-generated representation of aspirin.
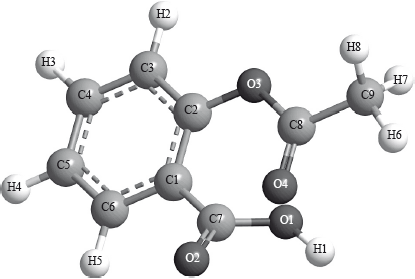
A third student measured selected bond lengths in aspirin, using this computer program and reported the following data.
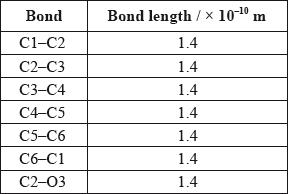
The following hypothesis was suggested by the student: “Since all the measured carbon-carbon bond lengths are equal, all the carbon-oxygen bond lengths must also be equal in aspirin. Therefore, the C8–O4 bond length must be 1.4 \( \times \) 10–10 m”. Comment on whether or not this is a valid hypothesis.
The other product of the reaction is ethanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\). Define an acid according to the Brønsted-Lowry theory and state the conjugate base of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\).
Brønsted-Lowry definition of an acid:
Conjugate base of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\):
A class studied the equilibrium established when ethanoic acid and ethanol react together in the presence of a strong acid, using propanone as an inert solvent. The equation is given below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}} + {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
One group made the following initial mixture:
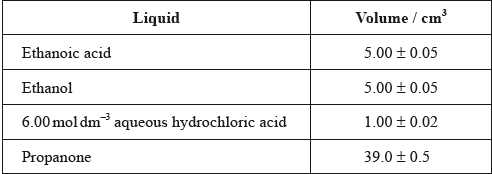
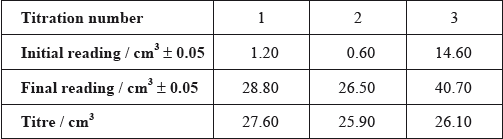
After one week, a \(5.00 \pm 0.05{\text{ c}}{{\text{m}}^{\text{3}}}\) sample of the final equilibrium mixture was pipetted out and titrated with \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 2}}\) aqueous sodium hydroxide to determine the amount of ethanoic acid remaining. The following titration results were obtained:
The density of ethanoic acid is \({\text{1.05 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\). Determine the amount, in mol, of ethanoic acid present in the initial mixture.
The hydrochloric acid does not appear in the balanced equation for the reaction. State its function.
Identify the liquid whose volume has the greatest percentage uncertainty.
(i) Calculate the absolute uncertainty of the titre for Titration 1 (\({\text{27.60 c}}{{\text{m}}^{\text{3}}}\)).
(ii) Suggest the average volume of alkali, required to neutralize the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample, that the student should use.
(iii) \({\text{23.00 c}}{{\text{m}}^{\text{3}}}\) of this \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide reacted with the ethanoic acid in the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample. Determine the amount, in mol, of ethanoic acid present in the \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of final equilibrium mixture.
Referring back to your answer for part (a), calculate the percentage of ethanoic acid converted to ethyl ethanoate.
Deduce the equilibrium constant expression for the reaction.
Outline how you could establish that the system had reached equilibrium at the end of one week.
Outline why changing the temperature has only a very small effect on the value of the equilibrium constant for this equilibrium.
Outline how adding some ethyl ethanoate to the initial mixture would affect the amount of ethanoic acid converted to product.
Propanone is used as the solvent because one compound involved in the equilibrium is insoluble in water. Identify this compound and explain why it is insoluble in water.
Suggest one other reason why using water as a solvent would make the experiment less successful.
Reaction kinetics can be investigated using the iodine clock reaction. The equations for two reactions that occur are given below.
Reaction A: \({{\text{H}}_2}{{\text{O}}_2}{\text{(aq)}} + {\text{2}}{{\text{I}}^ - }{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} \to {{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\)
Reaction B: \({{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{S}}_2}{\text{O}}_3^{2 - }{\text{(aq)}} \to {\text{2}}{{\text{I}}^ - }{\text{(aq)}} + {{\text{S}}_4}{\text{O}}_6^{2 - }{\text{(aq)}}\)
Reaction B is much faster than reaction A, so the iodine, \({{\text{I}}_{\text{2}}}\), formed in reaction A immediately reacts with thiosulfate ions, \({{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }\), in reaction B, before it can react with starch to form the familiar blue-black, starch-iodine complex.
In one experiment the reaction mixture contained:
\(5.0 \pm 0.1{\text{ c}}{{\text{m}}^{\text{3}}}\) of \({\text{2.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrogen peroxide \({\text{(}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{)}}\)
\(5.0 \pm 0.1{\text{ c}}{{\text{m}}^{\text{3}}}\) of 1% aqueous starch
\(20.0 \pm 0.1{\text{ c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sulfuric acid (\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\))
\(20.0 \pm 0.1{\text{ c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.0100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium thiosulfate (\({\text{N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}\))
\(50.0 \pm 0.1{\text{ c}}{{\text{m}}^{\text{3}}}\) of water with 0.0200 ± 0.0001 g of potassium iodide (KI) dissolved in it.
After 45 seconds this mixture suddenly changed from colourless to blue-black.
Calculate the amount, in mol, of KI in the reaction mixture.
Calculate the amount, in mol, of \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\) in the reaction mixture.
The concentration of iodide ions, \({{\text{I}}^ - }\), is assumed to be constant. Outline why this is a valid assumption.
For this mixture the concentration of hydrogen peroxide, \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\), can also be assumed to be constant. Explain why this is a valid assumption.
Explain why the solution suddenly changes colour.
Apart from the precision uncertainties given, state one source of error that could affect this investigation and identify whether this is a random error or a systematic error.
Calculate the total uncertainty, in \({\text{c}}{{\text{m}}^{\text{3}}}\), of the volume of the reaction mixture.
The colour change occurs when \(1.00 \times {10^{ - 4}}{\text{ mol}}\) of iodine has been formed. Use the total volume of the solution and the time taken, to calculate the rate of the reaction, including appropriate units.
In a second experiment, the concentration of the hydrogen peroxide was decreased to \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) while all other concentrations and volumes remained unchanged. The colour change now occurred after 100 seconds. Explain why the reaction in this experiment is slower than in the original experiment.
In a third experiment, 0.100 g of a black powder was also added while all other concentrations and volumes remained unchanged. The time taken for the solution to change colour was now 20 seconds. Outline why you think the colour change occurred more rapidly and how you could confirm your hypothesis.
Explain why increasing the temperature also decreases the time required for the colour to change.
Consider the following reaction taking place at 375 °C in a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) closed container.
\[\begin{array}{*{20}{l}} {{\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} + {\text{S}}{{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}}}&{\Delta {H^\Theta } = - 84.5{\text{ kJ}}} \end{array}\]
A solution of hydrogen peroxide, \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\), is added to a solution of sodium iodide, NaI, acidified with hydrochloric acid, HCl. The yellow colour of the iodine, \({{\text{I}}_{\text{2}}}\), can be used to determine the rate of reaction.
\[{{\text{H}}_2}{{\text{O}}_2}{\text{(aq)}} + {\text{2NaI(aq)}} + {\text{2HCl(aq)}} \to {\text{2NaCl(aq)}} + {{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\]
The experiment is repeated with some changes to the reaction conditions. For each of the changes that follow, predict, stating a reason, its effect on the rate of reaction.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
If the temperature of the reaction is changed to 300 °C, predict, stating a reason in each case, whether the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) and the value of \({K_{\text{c}}}\) will increase or decrease.
If the volume of the container is changed to \({\text{1.50 d}}{{\text{m}}^{\text{3}}}\), predict, stating a reason in each case, how this will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) and the value of \({K_{\text{c}}}\).
Suggest, stating a reason, how the addition of a catalyst at constant pressure and temperature will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\).
Graphing is an important method in the study of the rates of chemical reaction. Sketch a graph to show how the reactant concentration changes with time in a typical chemical reaction taking place in solution. Show how the rate of the reaction at a particular time can be determined.
The concentration of \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\) is increased at constant temperature.
The solution of NaI is prepared from a fine powder instead of large crystals.
Explain why the rate of a reaction increases when the temperature of the system increases.
A student used a pH meter to measure the pH of different samples of water at 298 K.
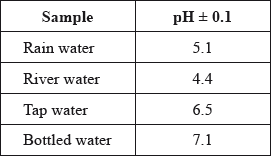
Use the data in the table to identify the most acidic water sample.
Calculate the percentage uncertainty in the measured pH of the rain water sample.
Determine the ratio of \({\text{[}}{{\text{H}}^ + }{\text{]}}\) in bottled water to that in rain water.
\[\frac{{[{H^ + }]{\text{ }}in{\text{ }}bottled{\text{ }}water}}{{[{H^ + }]{\text{ }}in{\text{ }}rain{\text{ }}water}}\]
The acidity of non-polluted rain water is caused by dissolved carbon dioxide. State an equation for the reaction of carbon dioxide with water.
Ethanedioic acid is a diprotic acid. A student determined the value of x in the formula of hydrated ethanedioic acid, \({\text{HOOC}}\)–\({\text{COOH}} \bullet {\text{x}}{{\text{H}}_{\text{2}}}{\text{O}}\), by titrating a known mass of the acid with a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of \({\text{NaOH(aq)}}\).
0.795 g of ethanedioic acid was dissolved in distilled water and made up to a total volume of \({\text{250 c}}{{\text{m}}^{\text{3}}}\) in a volumetric flask.
\({\text{25 c}}{{\text{m}}^{\text{3}}}\) of this ethanedioic acid solution was pipetted into a flask and titrated against aqueous sodium hydroxide using phenolphthalein as an indicator.
The titration was then repeated twice to obtain the results below.
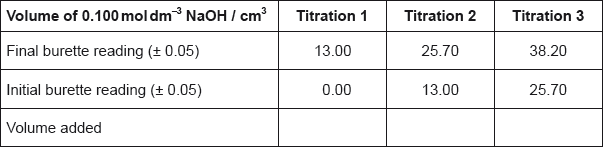
State the uncertainty of the volume of NaOH added in \({\text{c}}{{\text{m}}^{\text{3}}}\).
Calculate the average volume of NaOH added, in \({\text{c}}{{\text{m}}^{\text{3}}}\), in titrations 2 and 3, and then calculate the amount, in mol, of NaOH added.
(i) The equation for the reaction taking place in the titration is:
\({\text{HOOC}}\)−\({\text{COOH(aq)}} + {\text{2NaOH(aq)}} \to {\text{NaOOC}}\)−\({\text{COONa(aq)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\)
Determine the amount, in mol, of ethanedioic acid that reacts with the average volume of \({\text{NaOH(aq)}}\).
(ii) Determine the amount, in mol, of ethanedioic acid present in \({\text{250 c}}{{\text{m}}^{\text{3}}}\) of the original solution.
(ii) Determine the molar mass of hydrated ethanedioic acid.
(iv) Determine the value of x in the formula \({\text{HOOC}}\)−\({\text{COOH}} \bullet {\text{x}}{{\text{H}}_{\text{2}}}{\text{O}}\).
Identify the strongest intermolecular force in solid ethanedioic acid.
Deduce the Lewis (electron dot) structure of ethanedioic acid, HOOC−COOH.
The graph below shows pressure and volume data collected for a sample of carbon dioxide gas at 330 K.
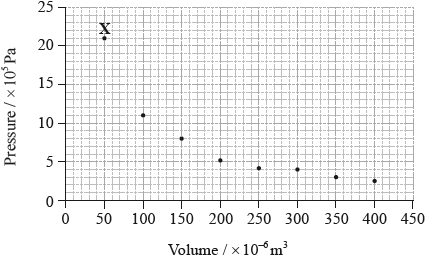
Draw a best-fit curve for the data on the graph.
Deduce the relationship between the pressure and volume of the sample of carbon dioxide gas.
Use the data point labelled X to determine the amount, in mol, of carbon dioxide gas in the sample.
A student carried out an experiment to determine the concentration of a hydrochloric acid solution and the enthalpy change of the reaction between aqueous sodium hydroxide and this acid by thermometric titration.
She added \({\text{5.0 c}}{{\text{m}}^{\text{3}}}\) portions of hydrochloric acid to \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution in a glass beaker until the total volume of acid added was \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\), measuring the temperature of the mixture each time. Her results are plotted in the graph below.

The initial temperature of both solutions was the same.
By drawing appropriate lines, determine the volume of hydrochloric acid required to completely neutralize the \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of sodium hydroxide solution.
Determine the concentration of the hydrochloric acid, including units.
Determine the change in temperature, \(\Delta T\).
Calculate the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction of hydrochloric acid and sodium hydroxide solution.
The accepted theoretical value from the literature of this enthalpy change is \( - 58{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Calculate the percentage error correct to two significant figures.
Suggest the major source of error in the experimental procedure and an improvement that could be made to reduce it.
The standard enthalpy change of three combustion reactions are given below.
\[\begin{array}{*{20}{l}} {{{\text{H}}_2}{\text{(g)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {{\text{H}}_2}{\text{O(l)}}}&{\Delta H = - 286{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{{\text{C}}_3}{{\text{H}}_8}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta H = - 2219{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{\text{C(s)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}}}&{\Delta H = - 394{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Determine the change in enthalpy, \(\Delta H\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the formation of propane in the following reaction.
\({\text{3C(s)}} + {\text{4}}{{\text{H}}_2}{\text{(g)}} \to {{\text{C}}_3}{{\text{H}}_8}{\text{(g)}}\)
A catalyst provides an alternative pathway for a reaction, lowering the activation energy, \({E_{\text{a}}}\). Define the term activation energy, \({E_{\text{a}}}\).
Sketch two Maxwell–Boltzmann energy distribution curves for a fixed amount of gas at two different temperatures, \({T_{\text{1}}}\) and \({T_2}{\text{ }}({T_2} > {T_1})\) and label both axes.

Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.
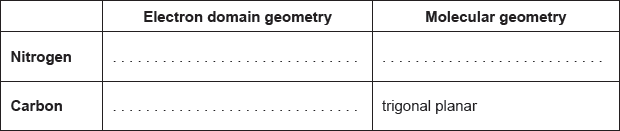
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) \( \rightleftharpoons \) (H2N)2CO(g) + H2O(g) ΔH < 0
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
The mass spectrum of urea is shown below.
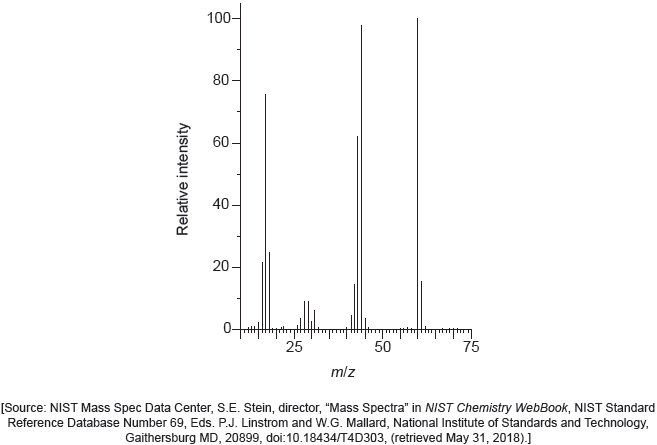
Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.
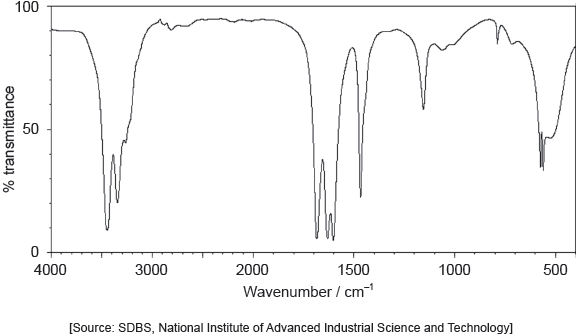
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
A student decided to determine the molecular mass of a solid monoprotic acid, HA, by titrating a solution of a known mass of the acid.
The following recordings were made.
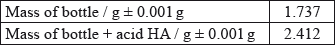
Calculate the mass of the acid and determine its absolute and percentage uncertainty.
This known mass of acid, HA, was then dissolved in distilled water to form a \({\text{100.0 c}}{{\text{m}}^{\text{3}}}\) solution in a volumetric flask. A \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) sample of this solution reacted with \({\text{12.1 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) NaOH solution. Calculate the molar mass of the acid.
The percentage composition of HA is 70.56% carbon, 23.50% oxygen and 5.94% hydrogen. Determine its empirical formula.
A solution of HA is a weak acid. Distinguish between a weak acid and a strong acid.
Describe an experiment, other than measuring the pH, to distinguish HA from a strong acid of the same concentration and describe what would be observed.
Airbags are an important safety feature in vehicles. Sodium azide, potassium nitrate and silicon dioxide have been used in one design of airbag.

Sodium azide, a toxic compound, undergoes the following decomposition reaction under certain conditions.
\[{\text{2Na}}{{\text{N}}_{\text{3}}}{\text{(s)}} \to {\text{2Na(s)}} + {\text{3}}{{\text{N}}_{\text{2}}}{\text{(g)}}\]
Two students looked at data in a simulated computer-based experiment to determine the volume of nitrogen generated in an airbag.
Using the simulation programme, the students entered the following data into the computer.
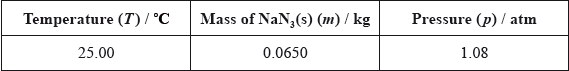
The chemistry of the airbag was found to involve three reactions. The first reaction involves the decomposition of sodium azide to form sodium and nitrogen. In the second reaction, potassium nitrate reacts with sodium.
\[{\text{2KN}}{{\text{O}}_3}{\text{(s)}} + {\text{10Na(s)}} \to {{\text{K}}_2}{\text{O(s)}} + {\text{5N}}{{\text{a}}_2}{\text{O(s)}} + {{\text{N}}_2}{\text{(g)}}\]
An airbag inflates very quickly.
Sodium azide involves ionic bonding, and metallic bonding is present in sodium. Describe ionic and metallic bonding.
State the number of significant figures for the temperature, mass and pressure data.
T:
m:
p:
Calculate the amount, in mol, of sodium azide present.
Determine the volume of nitrogen gas, in \({\text{d}}{{\text{m}}^{\text{3}}}\), produced under these conditions based on this reaction.
Suggest why it is necessary for sodium to be removed by this reaction.
The metal oxides from the second reaction then react with silicon dioxide to form a silicate in the third reaction.
\[{{\text{K}}_2}{\text{O(s)}} + {\text{N}}{{\text{a}}_2}{\text{O(s)}} + {\text{Si}}{{\text{O}}_2}{\text{(s)}} \to {\text{N}}{{\text{a}}_2}{{\text{K}}_2}{\text{Si}}{{\text{O}}_4}{\text{(s)}}\]
Draw the structure of silicon dioxide and state the type of bonding present.
Structure:
Bonding:
It takes just 0.0400 seconds to produce nitrogen gas in the simulation. Calculate the average rate of formation of nitrogen in (b) (iii) and state its units.
The students also discovered that a small increase in temperature (e.g. 10 °C) causes a large increase (e.g. doubling) in the rate of this reaction. State one reason for this.
The rate of the acid-catalysed iodination of propanone can be followed by measuring how the concentration of iodine changes with time.
I2(aq) + CH3COCH3(aq) → CH3COCH2I(aq) + H+(aq) + I−(aq)
Suggest how the change of iodine concentration could be followed.
A student produced these results with [H+] = 0.15 mol\(\,\)dm−3. Propanone and acid were in excess and iodine was the limiting reagent.
Determine the relative rate of reaction when [H+] = 0.15 mol\(\,\)dm−3.
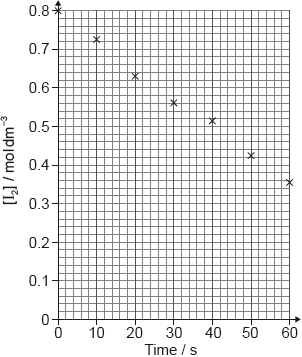
The student then carried out the experiment at other acid concentrations with all other conditions remaining unchanged.
State and explain the relationship between the rate of reaction and the concentration of acid.
Two groups of students (Group A and Group B) carried out a project* on the chemistry of some group 7 elements (the halogens) and their compounds.
* Adapted from J Derek Woollins, (2009), Inorganic Experiments and Open University, (2008), Exploring the Molecular World.
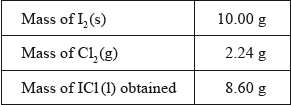
In the first part of the project, the two groups had a sample of iodine monochloride (a corrosive brown liquid) prepared for them by their teacher using the following reaction.
\[{{\text{I}}_{\text{2}}}{\text{(s)}} + {\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} \to {\text{2ICl(l)}}\]
The following data were recorded.
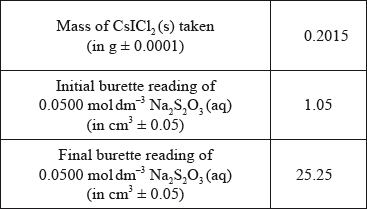
The students reacted ICl(l) with CsBr(s) to form a yellow solid, \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\), as one of the products. \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\) has been found to produce very pure CsCl(s) which is used in cancer treatment.
To confirm the composition of the yellow solid, Group A determined the amount of iodine in 0.2015 g of \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\) by titrating it with \({\text{0.0500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\). The following data were recorded for the titration.
(i) State the number of significant figures for the masses of \({{\text{I}}_{\text{2}}}{\text{(s)}}\) and ICl(l).
\({{\text{I}}_{\text{2}}}{\text{(s)}}\):
ICl (l):
(ii) The iodine used in the reaction was in excess. Determine the theoretical yield, in g, of ICl(l).
(iii) Calculate the percentage yield of ICl(l).
(iv) Using a digital thermometer, the students discovered that the reaction was exothermic. State the sign of the enthalpy change of the reaction, \(\Delta H\).
Although the molar masses of ICl and \({\rm{B}}{{\rm{r}}_2}\) are very similar, the boiling point of ICl is 97.4 °C and that of \({\rm{B}}{{\rm{r}}_2}\) is 58.8 °C. Explain the difference in these boiling points in terms of the intermolecular forces present in each liquid.
(i) Calculate the percentage of iodine by mass in \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\), correct to three significant figures.
(ii) State the volume, in \({\text{c}}{{\text{m}}^{\text{3}}}\), of \({\text{0.0500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\) used in the titration.
(iii) Determine the amount, in mol, of \({\text{0.0500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\) added in the titration.
(iv) The overall reaction taking place during the titration is:
\[{\text{CsICl(s)}} + {\text{2N}}{{\text{a}}_2}{{\text{S}}_2}{{\text{O}}_3}{\text{(aq)}} \to {\text{NaCl(aq)}} + {\text{N}}{{\text{a}}_2}{{\text{S}}_4}{{\text{O}}_6}{\text{(aq)}} + {\text{CsCl(aq)}} + {\text{NaI(aq)}}\]
Calculate the amount, in mol, of iodine atoms, I, present in the sample of \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\).
(v) Calculate the mass of iodine, in g, present in the sample of \({\text{CsIC}}{{\text{l}}_{\text{2}}}\)
(vi) Determine the percentage by mass of iodine in the sample of \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\), correct to three significant figures, using your answer from (v).
An acidic sample of a waste solution containing Sn2+(aq) reacted completely with K2Cr2O7 solution to form Sn4+(aq).
State the oxidation half-equation.
Deduce the overall redox equation for the reaction between acidic Sn2+(aq) and Cr2O72–(aq), using section 24 of the data booklet.
Calculate the percentage uncertainty for the mass of K2Cr2O7(s) from the given data.
The sample of K2Cr2O7(s) in (i) was dissolved in distilled water to form 0.100 dm3 solution. Calculate its molar concentration.
10.0 cm3 of the waste sample required 13.24 cm3 of the K2Cr2O7 solution. Calculate the molar concentration of Sn2+(aq) in the waste sample.
There are many oxides of silver with the formula AgxOy. All of them decompose into their elements when heated strongly.
After heating 3.760 g of a silver oxide 3.275 g of silver remained. Determine the empirical formula of AgxOy.
Suggest why the final mass of solid obtained by heating 3.760 g of AgxOy may be greater than 3.275 g giving one design improvement for your proposed suggestion. Ignore any possible errors in the weighing procedure.
Naturally occurring silver is composed of two stable isotopes, 107Ag and 109Ag.
The relative atomic mass of silver is 107.87. Show that isotope 107Ag is more abundant.
Some oxides of period 3, such as Na2O and P4O10, react with water. A spatula measure of each oxide was added to a separate 100 cm3 flask containing distilled water and a few drops of bromothymol blue indicator.
The indicator is listed in section 22 of the data booklet.
Deduce the colour of the resulting solution and the chemical formula of the product formed after reaction with water for each oxide.
Explain the electrical conductivity of molten Na2O and P4O10.
Outline the model of electron configuration deduced from the hydrogen line emission spectrum (Bohr’s model).
The structure of an organic molecule can help predict the type of reaction it can undergo.
Improvements in instrumentation have made identification of organic compounds routine.
The empirical formula of an unknown compound containing a phenyl group was found to be C4H4O. The molecular ion peak in its mass spectrum appears at m/z = 136.
The Kekulé structure of benzene suggests it should readily undergo addition reactions.
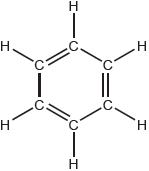
Discuss two pieces of evidence, one physical and one chemical, which suggest this is not the structure of benzene.
Formulate the ionic equation for the oxidation of propan-1-ol to the corresponding aldehyde by acidified dichromate(VI) ions. Use section 24 of the data booklet.
The aldehyde can be further oxidized to a carboxylic acid.
Outline how the experimental procedures differ for the synthesis of the aldehyde and the carboxylic acid.
Deduce the molecular formula of the compound.
Identify the bonds causing peaks A and B in the IR spectrum of the unknown compound using section 26 of the data booklet.
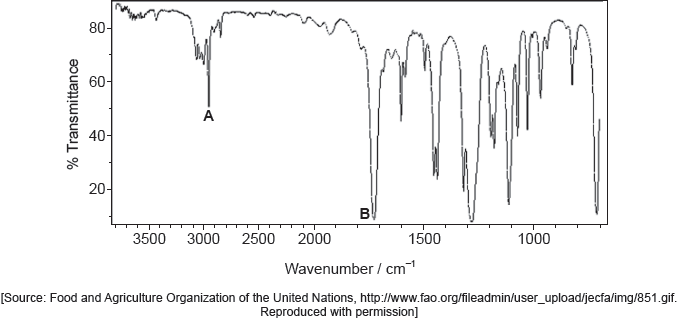
Deduce full structural formulas of two possible isomers of the unknown compound, both of which are esters.
Deduce the formula of the unknown compound based on its 1H NMR spectrum using section 27 of the data booklet.
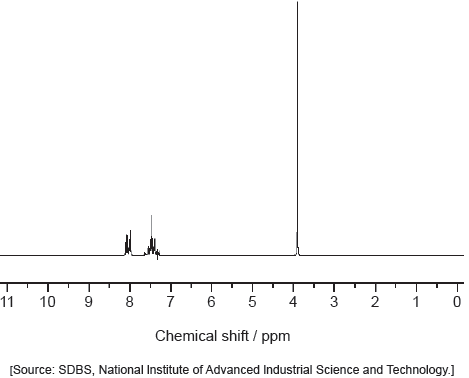
The reactivity of organic compounds depends on the nature and positions of their functional groups.
The structural formulas of two organic compounds are shown below.
Deduce the type of chemical reaction and the reagents used to distinguish between these compounds.
State the observation expected for each reaction giving your reasons.
Deduce the number of signals and the ratio of areas under the signals in the 1H NMR spectra of the two compounds.
Explain, with the help of equations, the mechanism of the free-radical substitution reaction of ethane with bromine in presence of sunlight.
The Bombardier beetle sprays a mixture of hydroquinone and hydrogen peroxide to fight off predators. The reaction equation to produce the spray can be written as:
| C6H4(OH)2(aq) + H2O2(aq) | → | C6H4O2(aq) + 2H2O(l) |
| hydroquinone | quinone |
Calculate the enthalpy change, in kJ, for the spray reaction, using the data below.
\(\begin{array}{*{20}{l}} {{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{{{\text{(OH)}}}_{\text{2}}}{\text{(aq)}} \to {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} + {{\text{H}}_{\text{2}}}{\text{(g)}}}&{\Delta {H^\theta } = + {\text{177.0 kJ}}} \\ {{\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}}}&{\Delta {H^\theta } = + {\text{189.2 kJ}}} \\ {{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {{\text{H}}_{\text{2}}}{\text{(g)}} + \frac{{\text{1}}}{{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(g)}}}&{\Delta {H^\theta } = + {\text{285.5 kJ}}} \end{array}\)
The energy released by the reaction of one mole of hydrogen peroxide with hydroquinone is used to heat 850 cm3 of water initially at 21.8°C. Determine the highest temperature reached by the water.
Specific heat capacity of water = 4.18 kJ\(\,\)kg−1\(\,\)K−1.
(If you did not obtain an answer to part (i), use a value of 200.0 kJ for the energy released, although this is not the correct answer.)
Identify the species responsible for the peak at m/z = 110 in the mass spectrum of hydroquinone.
Identify the highest m/z value in the mass spectrum of quinone.
This question is about carbon and chlorine compounds.
Ethane, C2H6, reacts with chlorine in sunlight. State the type of this reaction and the name of the mechanism by which it occurs.
Formulate equations for the two propagation steps and one termination step in the formation of chloroethane from ethane.
One possible product, X, of the reaction of ethane with chlorine has the following composition by mass:
carbon: 24.27%, hydrogen: 4.08%, chlorine: 71.65%
Determine the empirical formula of the product.
The mass and 1H\(\,\)NMR spectra of product X are shown below. Deduce, giving your reasons, its structural formula and hence the name of the compound.
Chloroethene, C2H3Cl, can undergo polymerization. Draw a section of the polymer with three repeating units.
Alkenes are widely used in the production of polymers. The compound A, shown below, is used in the manufacture of synthetic rubber.
(i) State the name, applying IUPAC rules, of compound A.
(ii) Draw a section, showing three repeating units, of the polymer that can be formed from compound A.
(iii) Compound A is flammable. Formulate the equation for its complete combustion.
Compound B is related to compound A.
(i) State the term that is used to describe molecules that are related to each other in the same way as compound A and compound B.
(ii) Suggest a chemical test to distinguish between compound A and compound B, giving the observation you would expect for each.
Test:
Observation with A:
Observation with B:
(iii) Spectroscopic methods could also be used to distinguish between compounds A and B.
Predict one difference in the IR spectra and one difference in the 1H NMR spectra of these compounds, using sections 26 and 27 of the data booklet.
IR spectra:
1H NMR spectra:
A sample of compound A was prepared in which the 12C in the CH2 group was replaced by 13C.
(i) State the main difference between the mass spectrum of this sample and that of normal compound A.
(ii) State the structure of the nucleus and the orbital diagram of 13C in its ground state.
Draw a 1s atomic orbital and a 2p atomic orbital.
A student titrated an ethanoic acid solution, CH3COOH (aq), against 50.0 cm3 of 0.995 mol dm–3 sodium hydroxide, NaOH (aq), to determine its concentration.
The temperature of the reaction mixture was measured after each acid addition and plotted against the volume of acid.
Curves X and Y were obtained when a metal carbonate reacted with the same volume of ethanoic acid under two different conditions.
Using the graph, estimate the initial temperature of the solution.
Determine the maximum temperature reached in the experiment by analysing the graph.
Calculate the concentration of ethanoic acid, CH3COOH, in mol dm–3.
Determine the heat change, q, in kJ, for the neutralization reaction between ethanoic acid and sodium hydroxide.
Assume the specific heat capacities of the solutions and their densities are those of water.
Calculate the enthalpy change, ΔH, in kJ mol–1, for the reaction between ethanoic acid and sodium hydroxide.
Explain the shape of curve X in terms of the collision theory.
Suggest one possible reason for the differences between curves X and Y.
Sodium thiosulfate solution reacts with dilute hydrochloric acid to form a precipitate of sulfur at room temperature.
Na2S2O3 (aq) + 2HCl (aq) → S (s) + SO2 (g) + 2NaCl (aq) + X
Identify the formula and state symbol of X.
Suggest why the experiment should be carried out in a fume hood or in a well-ventilated laboratory.
The precipitate of sulfur makes the mixture cloudy, so a mark underneath the reaction mixture becomes invisible with time.
10.0 cm3 of 2.00 mol dm-3 hydrochloric acid was added to a 50.0 cm3 solution of sodium thiosulfate at temperature, T1. Students measured the time taken for the mark to be no longer visible to the naked eye. The experiment was repeated at different concentrations of sodium thiosulfate.
Show that the hydrochloric acid added to the flask in experiment 1 is in excess.
Draw the best fit line of \(\frac{1}{{\rm{t}}}\) against concentration of sodium thiosulfate on the axes provided.
A student decided to carry out another experiment using 0.075 mol dm-3 solution of sodium thiosulfate under the same conditions. Determine the time taken for the mark to be no longer visible.
An additional experiment was carried out at a higher temperature, T2.
(i) On the same axes, sketch Maxwell–Boltzmann energy distribution curves at the two temperatures T1 and T2, where T2 > T1.
(ii) Explain why a higher temperature causes the rate of reaction to increase.
Suggest one reason why the values of rates of reactions obtained at higher temperatures may be less accurate.